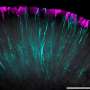
Researchers have made significant progress in the development of vascularized retinal organoids, which feature functional light-signal pathways. This breakthrough addresses a longstanding challenge in the field of regenerative medicine: maintaining retinal ganglion cells deep within organoids for extended periods. Traditional methods have struggled due to limited nutrient and oxygen supply in the densely packed tissues, leading to cell death.
The new approach, spearheaded by a team at the University of California, enhances the viability of retinal ganglion cells by incorporating vascular structures into the organoids. These structures enable a more efficient delivery of essential nutrients and oxygen, crucial for sustaining cellular health. The findings, published in March 2024, could pave the way for advanced treatments for retinal diseases.
Overcoming Nutrient Limitations
Maintaining the health of retinal ganglion cells within organoids has presented unique challenges. Previous research indicated that the dense arrangement of cells often restricts the diffusion of necessary substances, resulting in high rates of cell death. This limitation has hindered the potential applications of organoids in studying retinal diseases and developing therapeutic strategies.
By engineering vascularized structures, the research team has effectively created a microenvironment that mimics natural retinal tissue. These organoids not only support cell survival but also maintain functionality over time. As a result, they present an exciting opportunity for scientists and medical professionals alike to explore new avenues in vision restoration.
Implications for Regenerative Medicine
The implications of this research extend beyond basic science. The ability to produce viable retinal organoids holds promise for developing personalized treatments for conditions such as glaucoma and retinal dystrophies. By utilizing patients’ own cells to create these organoids, researchers can tailor therapies to individual needs, potentially increasing the efficacy of treatments.
Furthermore, the integration of functional light-signal pathways within these organoids allows for more precise studies of retinal function and disease mechanisms. This advancement may lead to new insights into how retinal cells respond to various stimuli, offering a clearer understanding of disease progression.
As this research develops, collaboration between academic institutions and biotechnology companies will be essential. The goal is to translate these findings into practical applications that can benefit patients suffering from vision-related disorders. The merging of engineering and biology in this context exemplifies the potential for innovative solutions in healthcare.
In summary, the development of vascularized retinal organoids marks a significant milestone in regenerative medicine. With improved nutrient supply and functionality, these organoids could revolutionize the way researchers study retinal diseases and develop targeted therapies, ultimately aiming to restore vision for those affected. The ongoing work at the University of California represents a promising step toward achieving these goals.