Research conducted at the University of Manchester has provided significant insights into how the immune system in the gut changes following a stroke and its potential impact on gastrointestinal health. Published in the journal Brain, Behavior, and Immunity, the study supports the concept of the “gut-brain axis,” suggesting a complex communication network between the gut and the brain that influences health and disease.
Understanding the effects of stroke is critical, as it is a life-threatening condition that disrupts blood flow to the brain, often resulting in long-term challenges related to mobility and cognition. Patients recovering from a stroke frequently face gastrointestinal complications, including difficulty swallowing and constipation. These issues are further complicated by the risk of secondary bacterial infections, which can be exacerbated by changes in the body’s microbiota—the community of beneficial bacteria that help maintain gut health.
The latest findings highlight that these gastrointestinal problems may be linked to alterations in commensal microbiota following a stroke. Previous research by some co-authors of this study has indicated that signals from the nervous system can influence gut immune responses post-stroke. The current study expands on this idea, revealing that the gut-brain axis may function bidirectionally. Specifically, the researchers observed that immune cells producing antibodies migrate to the brain and its membranes during a stroke. While the implications for stroke severity and prognosis remain unclear, this discovery opens new avenues for understanding the broader impact of stroke on overall health.
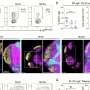
Using mouse models, the research team investigated changes in the small intestine after a stroke event. They documented that populations of immune cells responsible for producing antibodies showed significant alterations within just a few days. Notably, a specialized subset of these cells, which produce Immunoglobulin A (IgA), became hyper-activated. IgA plays a crucial role in managing the populations of beneficial bacteria in the gut, thereby impacting gut health.
The researchers found that mice lacking IgA did not experience the same degree of microbiota changes following a stroke. This suggests that alterations in immune function could partially explain the gastrointestinal symptoms observed in stroke patients. Lead investigator Professor Matt Hepworth from the Lydia Becker Institute of Immunity and Inflammation stated, “Stroke is a devastating neurological event but also has many long-term consequences that can leave the patient at risk of airway infection, as well as gastrointestinal complications.”
Professor Hepworth emphasized the importance of collaboration with neuroscientists in uncovering how gut immune systems become disturbed after a stroke, potentially leading to changes in how the gut interacts with its beneficial bacteria. “We now think these immune changes might contribute to the intestinal symptoms and long-term complications seen in stroke patients,” he added.
While current efforts focus on stroke prevention and minimizing damage through early intervention, this study highlights the importance of understanding the secondary health issues that arise throughout the body post-stroke. As immune-targeting therapeutics gain traction in clinical settings, this research paves the way for potential treatments aimed at alleviating immune-driven gastrointestinal symptoms following a stroke, ultimately improving patients’ quality of life.
More information on this study can be found in the article by Madeleine Hurry et al, titled “Cerebral ischaemic stroke results in altered mucosal antibody responses and host-commensal microbiota interactions,” published in Brain, Behavior, and Immunity in 2026. The DOI for the study is 10.1016/j.bbi.2025.106184.