In a significant breakthrough for pancreatic cancer treatment, a new drug known as daraxonrasib is showing promise in clinical trials at Penn Medicine’s Abramson Cancer Center. This drug, a type of KRAS inhibitor, aims to block a protein critical for the growth of this aggressive cancer, which has one of the highest mortality rates among all cancers.
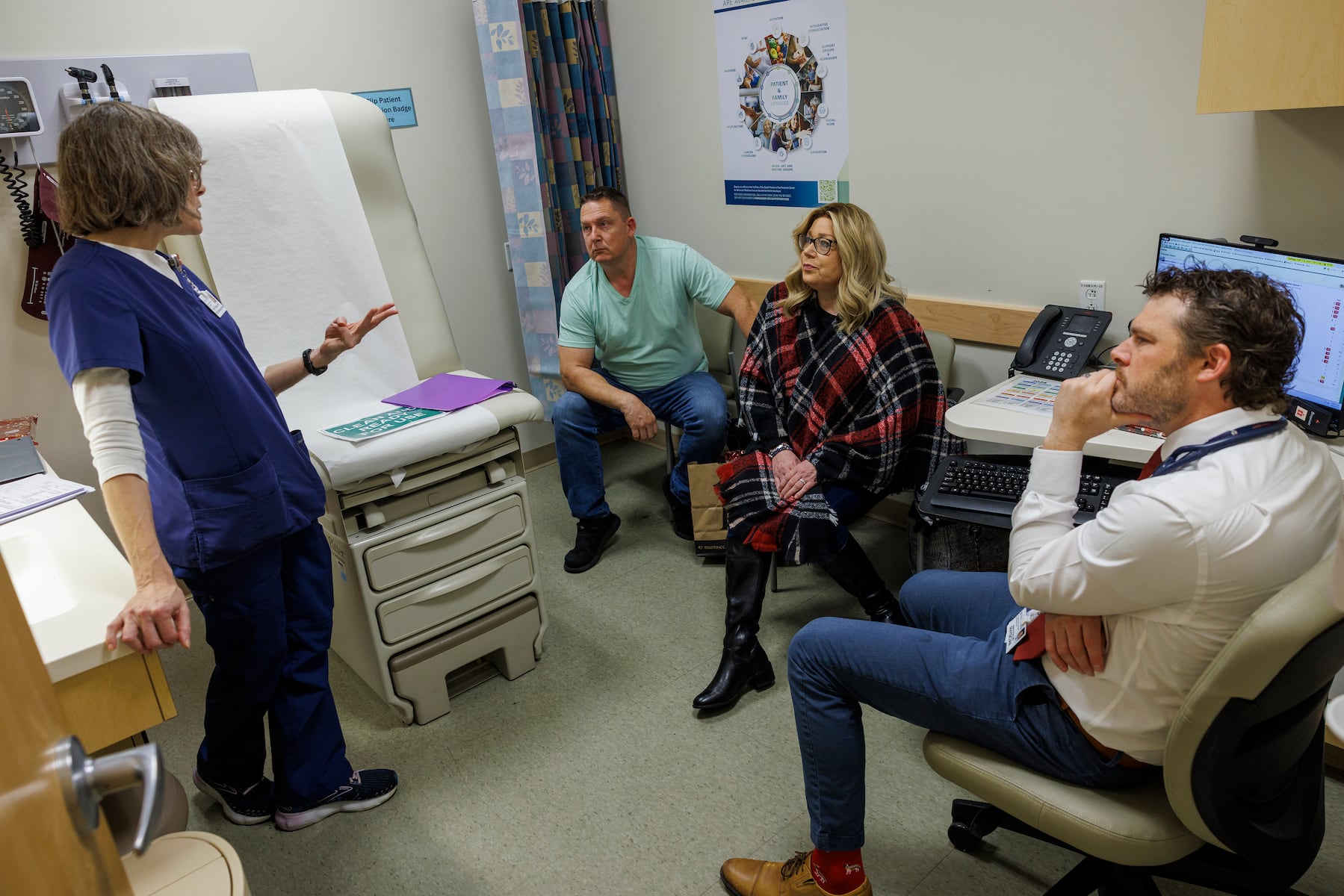
In June, Irene Blair, a 59-year-old grandmother from Newark, Delaware, was given a prognosis of only six to eight months to live after her pancreatic cancer advanced to stage 4. With limited options available, she enrolled in a clinical trial for daraxonrasib, which is considered a hopeful advancement in cancer therapies.
The urgency around this drug has intensified following the recent announcement by former Nebraska Senator Ben Sasse, who revealed his diagnosis of stage-four pancreatic cancer. His candid disclosure highlights the dire need for effective treatments as he faces the reality of this disease.
Clinical trial results indicate that daraxonrasib may significantly extend survival times. In a phase 1 trial involving 38 patients, the drug reportedly doubled survival duration for approximately half of the participants, increasing their median survival from seven months to 15.6 months compared to standard chemotherapy.
Mark O’Hara, Blair’s oncologist at Penn Medicine, emphasized the lack of effective therapies for pancreatic cancer beyond chemotherapy. “For too long, we haven’t had effective therapies beyond just chemotherapy,” he stated. Following her initial treatment with daraxonrasib in July, Blair experienced a remarkable reduction in cancer-related pain within three weeks, and subsequent scans showed her tumors were stable or decreasing.
The development of KRAS inhibitors has been a long-time goal for cancer researchers since the discovery of the KRAS protein in 1982. This mutated protein accelerates tumor growth, and such mutations are present in approximately 25% of all human cancers. The first FDA-approved KRAS inhibitors emerged in 2021 for lung cancer, paving the way for research into treatments for pancreatic cancer, where nearly 90% of cases involve KRAS mutations.
Daraxonrasib, also classified as a “pan-RAS inhibitor,” targets not only KRAS but also two other related proteins, HRAS and NRAS. In recent trials, over 90% of participants experienced stalled cancer progression, and around 30% saw their tumors shrink.
The oral medication, taken in the form of three daily pills, has manageable side effects. The most commonly reported is a rash, experienced by 91% of trial participants, with 8% suffering severe cases. Other side effects include diarrhea, nausea, and mouth sores, which are generally manageable with medication. O’Hara noted that the overall quality of life for patients on daraxonrasib is often better than that experienced on traditional chemotherapy.
For Blair, the improvement in her health allows her to focus on living her life. After retiring from her real estate career in May, she looks forward to traveling and spending time with family. “You just wonder, ‘Will I be here next year?’” she reflected, expressing the emotional weight of her diagnosis.
As research into KRAS inhibitors progresses, the medical community remains hopeful that daraxonrasib could herald a new era in the treatment of pancreatic cancer, providing patients with more time and improved quality of life.