A recent study has revealed a critical molecular mechanism that enhances our understanding of how cells communicate through extracellular vesicles (EVs). Published in the Journal of Extracellular Vesicles on November 28, 2025, this research highlights the role of the Commander protein complex, which is known for its involvement in membrane recycling. This complex also plays a significant role in coordinating the entry and internal destination of vesicles within cells, thus advancing our knowledge of intercellular communication.
The study was led by Professor Albert Lu from the Faculty of Medicine and Health Sciences at the University of Barcelona and the CELLEX Biomedical Research Center (IDIBAPS-UB), alongside María Yáñez-Mó from the Severo Ochoa Center for Molecular Biology (CSIC-UAM). Their findings are particularly relevant for the development of innovative therapies and diagnostic tools that could harness the therapeutic potential of EVs.
Understanding the mechanics of how receptor cells capture and process extracellular vesicles is vital, according to Professor Lu. He stated, “This knowledge is key to harnessing the therapeutic and diagnostic potential of these vesicles, since their effectiveness depends on being able to direct them and have them captured by the appropriate target cells.”
Innovative Approach Using CRISPR Technology
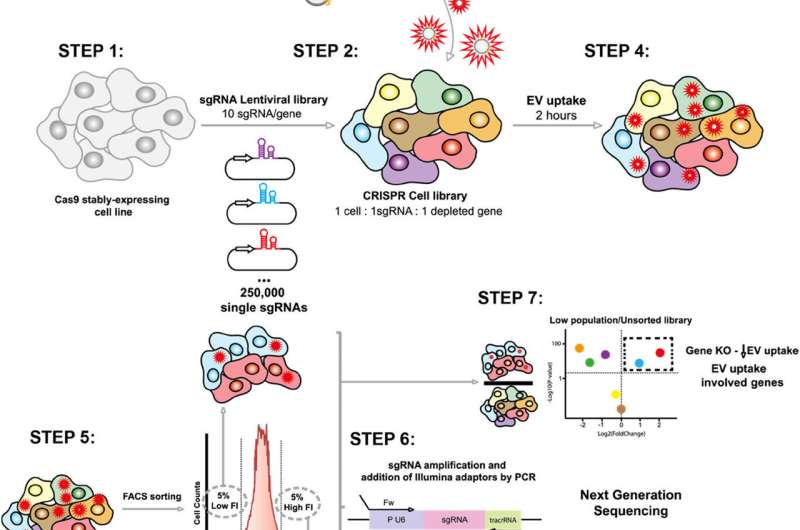
To investigate the molecular mechanisms guiding the uptake of EVs, the research team employed a groundbreaking methodology based on CRISPR-Cas9 technology. This genomic screening method allows for the deactivation of each of the more than 20,000 human genes individually, enabling researchers to analyze their specific roles in the uptake process.
In the study, cells were genetically modified so that each group had a different gene deactivated. These modified cells were then exposed to EVs labeled with a fluorescent dye. Using flow cytometry, researchers measured which cells captured more or fewer vesicles. Subsequent analysis using fluorescence-activated cell sorting (FACS) allowed for the separation of cells based on their uptake capacity. The deactivated genes were identified through mass sequencing.
“This systematic and unbiased approach allows us to discover new regulators without relying on prior hypotheses,” Professor Lu explained. The findings indicate that the Commander endosomal recycling complex, composed of various proteins, serves as a fundamental regulator of vesicle uptake. The research suggests that this mechanism is conserved across different human cell lines, although its activity may vary depending on cell type or physiological context.
Implications for Future Therapies
The implications of understanding EV communication extend beyond basic science to therapeutic applications. The ability of EVs to cross membranes and target specific tissues positions them as potential natural vehicles for delivering drugs or therapeutic molecules. “Understanding how their entry, intracellular trafficking, and delivery of their molecular cargo are regulated opens the door to designing EVs with controlled directionality,” said Professor Lu, highlighting their potential in regenerative, oncological, or anti-inflammatory therapies.
Researchers are currently focused on gaining a deeper understanding of the Commander complex’s role in controlling EV uptake and their intracellular fate. They aim to explore whether similar mechanisms are applicable in other cell types or tissues and investigate how alterations in this complex might affect cell communication in pathological contexts, such as cancer or neurodegenerative disorders.
Professor Lu concluded, “In the long term, the goal is to manipulate this pathway to modulate communication between cells and enhance the therapeutic and diagnostic applications of EVs.”
This groundbreaking research not only enhances our understanding of cellular communication but also opens new avenues for the development of targeted therapies that could significantly impact the treatment of various diseases. Further studies will undoubtedly continue to unravel the complexities of EVs and their potential in medicine.