A new intranasal vaccine developed by researchers at Chiba University could offer a groundbreaking treatment option for cervical cancer. While the existing human papillomavirus (HPV) vaccines are effective in preventing infections, they do not eliminate existing ones. This innovative approach, detailed in a study published in Science Translational Medicine, aims to activate local immune responses to inhibit the progression of cervical cancer.
Led by Rika Nakahashi-Ouchida, MD, and Hiromi Mori of Chiba University Hospital, the research focuses on a novel nanogel-based vaccine that targets the E7 oncoprotein produced by high-risk HPV strains, particularly HPV16. This protein is known to deactivate the tumor suppressor pRb, leading to cervical cancer development.
Cervical cancer is a significant global health issue, with approximately 670,000 new cases and 350,000 deaths reported worldwide in 2022, according to the World Health Organization. The burden is especially heavy in low- and middle-income countries, where access to HPV vaccination and treatment options is limited. Current therapies, including surgery and chemotherapy, can have serious side effects, including fertility loss.
To address these pressing needs, the research team developed a nasal vaccine utilizing cationic cholesteryl-group-bearing nanogels (cCHP) to deliver HPV antigens directly to the nasal mucosa. These nanogels, which are positively charged, adhere effectively to the negatively charged surfaces of the nasal cavity, allowing for a gradual release of the antigen.
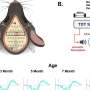
The vaccine was formulated with the E7 antigen and cyclic-di-AMP (c-di-AMP), an adjuvant that enhances T-cell-mediated immunity. When administered intranasally to mice and macaques, the vaccine demonstrated promising results. In mice, it significantly slowed tumor growth and induced E7-specific CD4+ and CD8+ T cells in the cervicovaginal tissue. In macaques, four doses delivered via a compatible nasal spray device resulted in robust immune responses, maintaining high levels of E7-specific killer T cells up to four months post-treatment.
Nakahashi-Ouchida remarked, “We have developed an intranasal therapeutic vaccine as a nonsurgical alternative to conventional treatments that can compromise women’s quality of life.” This novel delivery method activates mucosal homing pathways for lymphocytes, which enhances immune responses specifically in the cervical mucosa.
The findings suggest a significant potential for this nasal delivery system to stimulate mucosal immunity within the reproductive tract, leveraging the respiratory-reproductive axis—a concept previously validated in other viral models by the research team. Importantly, the vaccine’s success in inducing localized immune responses in primates provides a strong basis for its future clinical applications.
As researchers continue to explore therapeutic options for HPV-related conditions, the work from Chiba University emphasizes the transformative potential of nanogel-based vaccines. By offering a non-invasive treatment that preserves fertility and improves patients’ quality of life, this approach could redefine cervical cancer management.
Nakahashi-Ouchida added, “Immunotherapies such as intranasal therapeutic vaccines may help establish a new category of noninvasive treatment. These approaches could extend to recurrence prevention and chronic disease management, providing patients with safer and more accessible options.” While further clinical trials are necessary to validate these findings, the research represents an important advancement in extending the role of immunotherapy from prevention to treatment, paving the way for a new generation of mucosal-targeted vaccines.