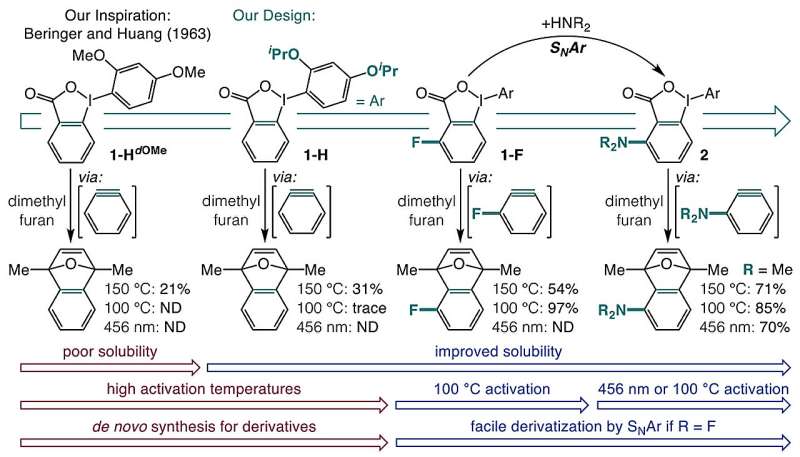
A research team from the University of Minnesota has introduced a groundbreaking one-pot method for synthesizing blue light-responsive aryne precursors from carboxylic acids. This innovative approach simplifies the previously complex process of generating arynes, which are highly reactive organic intermediates essential in the synthesis of complex aromatic molecules used in pharmaceuticals and agricultural chemistry.
The findings, published in the journal Nature, reveal that the new method enables the creation of numerous previously unreported aminated arynes and 20 entirely new aryne precursors in a single step through nucleophilic aromatic substitution (SNAr).
Challenges with Traditional Aryne Synthesis
Arynes possess a unique structure characterized by a triple bond within an aromatic ring, granting them the ability to react with a variety of functional groups. Despite their potential, synthetic chemists have faced significant challenges in utilizing arynes in organic reactions. Traditional synthesis methods often involve harsh bases to remove protons from strong C–H bonds, followed by halide elimination. This complexity limits their use, particularly with sensitive functional groups.
Previous attempts to use thermally activated precursors have resulted in highly explosive compounds, while ultraviolet (UV) light techniques frequently led to unintended reactions. These challenges underscored the need for a simpler and milder approach to generating diverse aryne derivatives from readily available materials.
A New Era of Aryne Accessibility
To tackle this long-standing issue, the research team focused on the potential of o-iodoniobenzoates as a more straightforward pathway to aryne precursors. Initially, they encountered solubility issues and unwanted side reactions. However, through iterative experimentation, they found that incorporating isopropoxy groups significantly enhanced solubility and reduced these adverse effects.
The final breakthrough involved adding a substituent adjacent to the carboxylate group, producing aryne precursors that respond to either blue light activation or mild heat at 100 °C. The mechanism of activation varies; heat triggers a field effect where nearby chemical groups induce decarboxylation, while blue light at 398 nm excites the molecule into a triplet state, causing the aromatic ring to cleave from iodine and lose carbon dioxide, thus forming the aryne.
This novel one-pot synthesis method unlocks access to a vast array of aryne precursors, compatible with various functional groups. The researchers emphasize that this advancement not only simplifies the synthesis of complex aromatic compounds but also opens unexplored avenues in chemical research, particularly for drug discovery and agrochemicals.
The study, authored by Chris M. Seong and colleagues, represents a significant step forward in synthetic chemistry, allowing chemists to generate arynes more efficiently and safely. It highlights the importance of innovative approaches in overcoming long-standing barriers in chemical synthesis, paving the way for new applications in various fields.