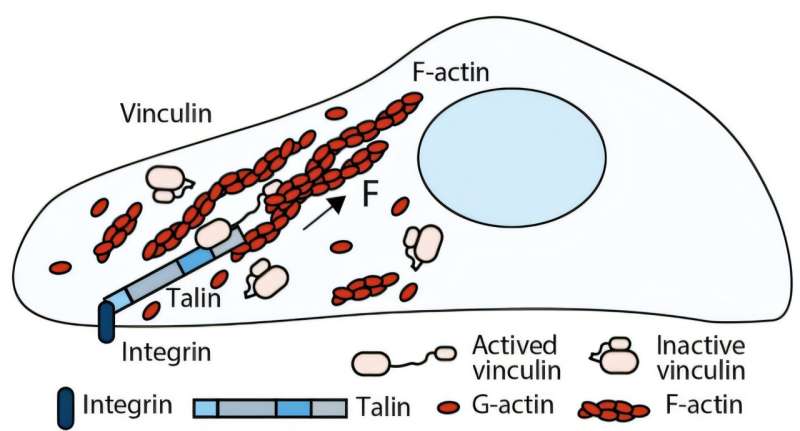
A team of researchers from the University of Liverpool, in collaboration with the Mechanobiology Institute at the National University of Singapore, has made a groundbreaking discovery regarding cellular response to physical forces. Their study, published in Science Advances on November 3, 2025, reveals that vinculin, a protein previously viewed as a mere structural component, actively participates in mechanical signaling and cellular memory.
Traditionally, vinculin was considered primarily a structural link between adhesion complexes and the cytoskeleton. However, the new research indicates that vinculin features six force-dependent binary switches, which function similarly to mechanical memory. Utilizing single-molecule magnetic tweezers, the researchers successfully manipulated individual vinculin molecules, providing a detailed characterization of these switches. This finding represents a significant shift in the field of mechanobiology, enhancing the understanding of how cells process mechanical signals.
Implications for Health and Neuroscience
Professor Ben Goult, who specializes in Mechanistic Cell Biology at the University of Liverpool, emphasized the importance of this discovery. “Our finding that vinculin is mechanically active opens up a new area of research. These switches suggest that vinculin is not just a structural component, but a dynamic participant in cellular decision-making. It’s a fundamental change in how we view this protein,” he stated.
The implications extend beyond basic research. Mutations in vinculin are associated with dilated cardiomyopathy and heart failure. Reevaluating these mutations through the lens of vinculin’s mechanical switches could enhance the understanding of disease mechanisms and identify potential therapeutic targets.
Furthermore, the study lays the groundwork for future research into vinculin’s role in the brain. The combined functions of talin and vinculin create a network of binary switches—termed the MeshCODE—that may be essential for processing and storing mechanical and chemical information in neurons. Collaborations with the Liverpool Interdisciplinary Neuroscience Center and the University of Helsinki aim to explore vinculin’s involvement in synaptic activity.
Future Research Directions
Although the current findings are based on in vitro experiments, the research team is also investigating vinculin in living cells and engineered heart tissues. Additionally, they are collaborating with the University of Liverpool’s Center for Proteome Research to study vinculin’s interactions and post-translational modifications during cell migration.
Professor Goult expressed optimism about the future of mechanobiology, stating, “This is an exciting time for mechanobiology. We’re beginning to see how mechanical forces shape cellular behavior in ways we hadn’t imagined. Vinculin’s switches may be the key to unlocking how cells remember and respond to their physical environment.”
For further details, refer to the article by Xuyao Liu et al., titled “The mechanical response of vinculin,” published in Science Advances. The DOI for the study is 10.1126/sciadv.ady6949.