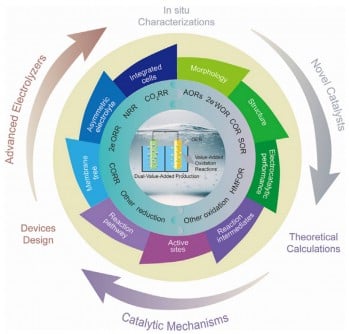
URGENT UPDATE: A groundbreaking study released by a research team from Jiangsu University, the Chinese Academy of Sciences, Hasselt University, and MIT reveals a transformative approach in electrolysis that could redefine sustainable energy production. Published online in eScience in July 2025, this comprehensive review highlights new techniques that replace traditional, inefficient oxygen evolution reactions (OER) with value-added processes, marking a pivotal shift towards cleaner and more efficient energy solutions.
This innovative research addresses the urgent need to reduce reliance on fossil fuels, which currently account for over 80% of global energy consumption and significantly contribute to rising CO2 emissions. As climate change and energy insecurity escalate, this study provides a timely roadmap for integrating renewable energy sources into chemical processes.
The review underscores the potential of electrosynthesis in achieving dual outputs—clean fuels and market-relevant chemicals—while dramatically lowering energy consumption. By pairing alternative oxidation reactions with reduction processes, the research team demonstrates how to enhance system efficiency and produce valuable by-products like formic acid and hydrogen peroxide.
Prof. Zhenhai Wen, one of the co-authors, emphasized the importance of these advancements: “Electrochemical systems that simultaneously produce two valuable outputs represent a paradigm shift for green chemistry.” This innovative approach is critical for addressing both environmental and economic challenges, making it a compelling development for industries looking to adopt greener practices.
Significant breakthroughs in catalyst development are at the core of this research. The study reveals advancements in nanostructured materials that increase active sites and optimize selectivity, enhancing the performance of electrosynthesis systems. The introduction of self-supported and gas-diffusion electrodes is also highlighted, contributing to improved stability and conversion rates.
The researchers also discuss the evolution of hybrid electrolyzers, which are transitioning from traditional H-type cells to more efficient flow cells and membrane electrode assemblies. This evolution enables industrial-scale current densities, making the technology more viable for widespread adoption.
Additional insights come from the incorporation of advanced monitoring techniques, including infrared spectroscopy and electron microscopy. These tools allow researchers to observe catalytic processes in real-time, providing a deeper understanding of reaction mechanisms and facilitating the design of more effective catalysts.
As the world grapples with the challenges of climate change, the implications of this research are profound. The development of dual-value electrosynthesis systems not only promises a reduction in carbon emissions but also enables the production of green hydrogen, fertilizers, and chemical feedstocks at a lower cost. This makes it a significant step towards a sustainable and circular chemical industry.
The study is supported by various funding bodies, including the National Natural Science Foundation of China, and reflects a collaborative effort in advancing the field of electrochemistry.
In conclusion, the findings from this review may catalyze a shift towards sustainable practices in energy and chemical production, aligning with global net-zero ambitions. As industries look to reduce their carbon footprint, the integration of these innovative electrochemical systems will be crucial. Stay tuned for further developments as this research unfolds, potentially reshaping the future of green energy.